New bio-inspired molecule captures solar energy and releases it as heat on demand, outperforming lithium-ion batteries in energy density.
When the sun goes down, solar panels stop working. It is the fundamental hurdle of renewable energy: how to save the sun’s power for a rainy day — or a cold night.
Chemists at UC Santa Barbara have developed a solution that doesn’t require bulky batteries or electrical grids. In a paper published Feb. 12 in the journal Science, Associate Professor Grace Han and her team detail a new material that captures sunlight, stores it within the bonds of a molecule and releases it as heat on demand.
The material, a modified organic molecule called pyrimidone, is the latest advancement in Molecular Solar Thermal (MOST) energy storage.
“The concept is reusable and recyclable. Think of photochromic sunglasses. When you’re inside, they’re just clear lenses. You walk out into the sun, and they darken on their own. Come back inside, and the lenses become clear again. That kind of reversible change is what we’re interested in. Only instead of changing color, we want to use the same idea to store energy, release it when we need it, and then reuse the material over and over.”
— Han Nguyen, Ph.D. student in the Han Group
A ‘rechargeable battery’ for heat
While traditional solar panels convert light into electricity, MOST systems convert light into chemical energy. The molecule acts like a mechanical spring: when hit with sunlight, it twists into a strained, high-energy shape (an isomer). It stays locked in that shape until a trigger — such as a small amount of heat or a catalyst — snaps it back to its relaxed state, releasing the stored energy as heat.
“We typically describe it as a rechargeable solar battery,” Nguyen said. “It stores sunlight, and it can be recharged.”
The team’s new molecule is a heavy hitter. It boasts an energy density of more than 1.6 megajoules per kilogram. For context, that is roughly double the energy density of a standard lithium-ion battery (approx. 0.9 MJ/kg) and significantly higher than previous generations of optical switches.
From theory to boiling water
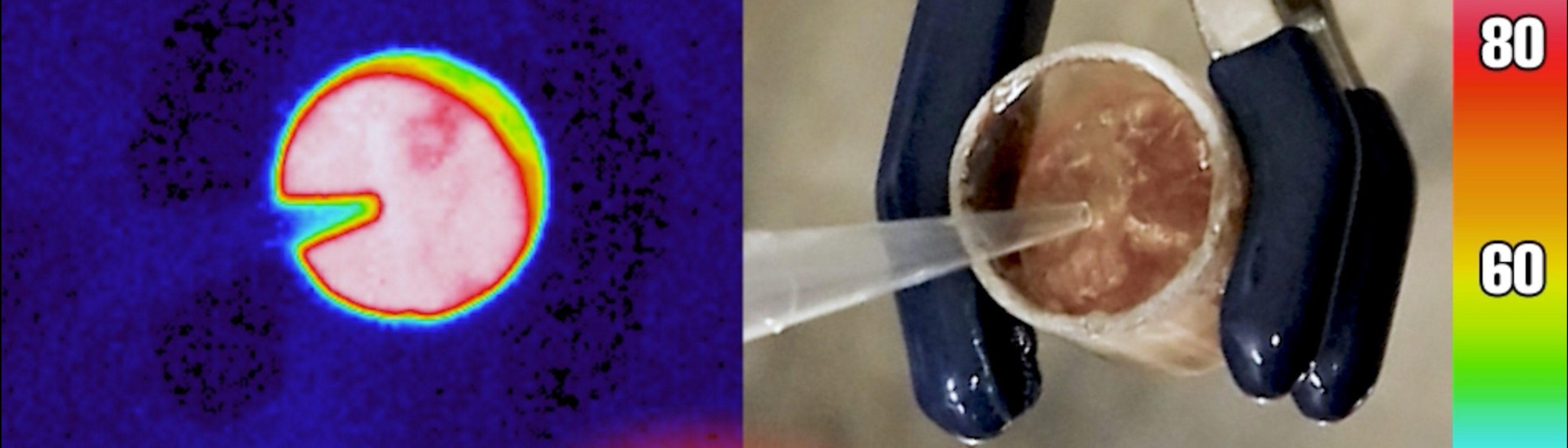
The critical breakthrough for Han’s group was translating high energy density into a tangible result. In the study, the researchers demonstrated that the heat released from the material was intense enough to boil water — a feat previously difficult to achieve in this field.
Thermal and digital cameras capture the moment water boiled by molecules that release heat upon activation. The molecules were pre-charged by light. Video shown at 10% real-time speed. Credit: Han P. Q. Nguyen, Grace G. D. Han Group, University of California, Santa Barbara.
“Boiling water is an energy-intensive process,” Nguyen said. “The fact that we can boil water under ambient conditions is a big achievement.”
This capability opens the door for practical applications ranging from off-grid heating for camping to residential water heating. Because the material is soluble in water, it could potentially be pumped through roof-mounted solar collectors to charge during the day and stored in tanks to provide heat at night.
“With solar panels, you need an additional battery system to store the energy,” said co-author Benjamin Baker, a Ph.D. student in the Han Lab. “With molecular solar thermal energy storage, the material itself is able to store that energy from sunlight.”
Bio-inspired design
To create this molecule, the team looked to a surprising source: DNA. The pyrimidone structure is similar to a component found in DNA that, when exposed to UV light, can undergo reversible structural changes.
By engineering a synthetic version of this structure, the team created a molecule that stores and releases energy reversibly. They collaborated with Ken Houk, a distinguished research professor at UCLA, to use computational modeling to understand why the molecule was able to store energy and remain stable for years without losing the stored energy.
“We prioritized a lightweight, compact molecule design,” Nguyen said. “For this project, we cut everything we didn’t need. Anything that was unnecessary, we removed to make the molecule as compact as possible.”