Content:
Wet adhesion is a true engineering challenge. Marine animals such as mussels, oysters and barnacles are naturally equipped with the means to adhere to rock, buoys and other underwater structures and remain in place no matter how strong the waves and currents.
Synthetic wet adhesive materials, on the other hand, are a different story.
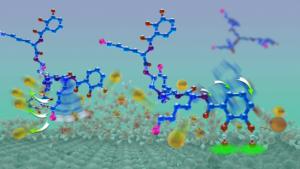
Taking their cue from Mother Nature and the chemical composition of mussel foot proteins, the Alison Butler Lab at UC Santa Barbara decided to improve a small molecule called the siderophore cyclic trichrysobactin (CTC) that they had previously discovered. They modified the molecule and then tested its adhesive strength in aqueous environments. The result: a compound that rivals the staying power of mussel glue.
Their findings appear today in the journal Science.
"There's real need in a lot of environments, including medicine, to be able to have glues that would work in an aqueous environment," said co-author Butler, a professor in UCSB's Department of Chemistry and Biochemistry. "So now we have the basis of what we might try to develop from here."